Lens geometry in STEM mode on a TEM
In normal TEM mode, we have two cross-overs below C2, the
lower one occurring within the objective pre-field, as shown
in the elementary guide. One way of forming
a probe would be to de-excite C2, as we did in the very
first experiment in first section of the TEM guide, but then we still have a
second cross-over which is happening somewhere odd within
the pre-field.
Greater flexibility and an optimised probe focus can be
obtained by avoiding the cross-over within the objective pre-
field. In practice, this is achieved by either lowering the overall
objective excitation or (depending on the make of microscope) using
another small lens
within the upper part of the objective twin lens, which is
called either a mini-condenser or mini-lens. When the
machine is being run in normal TEM mode, this lens is run in
the same sense as the objective, making the pre-field very
strong. When STEM mode is selected, the mini-lens is
reversed (or switched off), and so it cancels out the pre-field to a certain
extent.
The next Figure illustrates conventional TEM imaging:
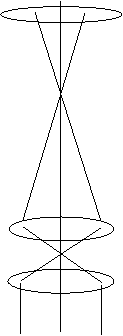
where the top lens is C2, and the two lower lenses represent the
objective prefield (and/or the objective lens pre-field boosted by
a condenser mini-lens or objective mini-lens).
Now when we go into STEM mode, most microscopes reduce the influence
of the pre-field, so now the ray-diagram looks like this:

Note that both C2 and the objective lens affect the
focus of the electron probe. Because C2 is so weakly excited,
you can see from the figure above that it will have a huge effect on
the convergence angle of the illumination at the specimen plane. For
this reason, many manufacturers fix the value of C2 in STEM mode.
To obtain control of it, the user has to override the computer.
Remember: In STEM, the only electron optics that matter all
happen before the specimen. Alignments you made relating to
TEM imaging, especially the objective focus and stigmation,
are useless in STEM mode. We must alter the objective lens (thus
altering the pre-field above the speciment) and C2 to control
the alignment. The relevant stigmators for STEM mode are the
condenser stigmators, not the objective stigmators.
Now that we know that two lenses are involved in the forming
probe, we can work how to line them up with one another.
The most sensitive mis-alignment arises from the double-
deflection coils being on the wrong tilt setting. If the
beam tilt is wrong, the beam from C2 passes into the
objective pre-field off-axis and at an angle. It will come
out below the specimen also at the wrong angle: this is most
common reason for losing the beam in STEM or nanoprobe mode
at high camera lengths.
The first thing to get right in STEM mode is therefore the
objective rotation centre. The alignment is almost
certainly not the same as for TEM, because the objective is
in a completely different state, and we are aligning with
respect to the pre-field, not the main body of the lens
below the specimen. Similarly, the condenser aperture is
almost certainly off-line, even if you lined it up in TEM
mode.
All the corrections are best done in the electron
Ronchigram.
Experiment: Wobble the objective lens, and try to get the
image moving in and out symmetrically with the condenser (we
assume here that this is on line). As you alter the beam tilt
(this should be the correction you are adjusting on the
multi-function knobs when aligning in STEM mode),
the whole Ronchigram will at the same time bodily move
across the phosphor screen. That's because the conical beam
cast by the condenser lens is rocking like a lever through
the specimen, and moves all over the far-field plane, like
the sweep of a torch beam. Start with a large condenser
aperture. The aim is to get a stationary shadow image at
the very centre of the disc you can see on the screen.
Just get the rotation centre roughly right, stop the
objective wobbling, and then change C2. (Remember that on
most machines you may have over-ride the C2 setting).
Is the Ronchigram going in and out symmetrically around a
point at the centre of the condenser aperture? If not,
shift the condenser aperture onto that axis. If in any
doubt at all, leave the condenser aperture where it was
correct for TEM imaging. You will not get a perfect STEM
image, but neither will you lose the beam, which is very
easy at this stage.
Now go back and wobble the objective lens again. When both
these centres are roughly co-incident with the condenser
aperture, set C2 at the value you are going to use it in
STEM mode.
Focus the objective to get the Ronchigram to 'blow up' into
infinitive magnification at its centre. Adjust the
condenser stigmators so that the Ronchigram changes
symmetrically as a function of defocus. This takes some
practice. (Remember that the objective stigmators, which
are below the specimen, are useless in STEM mode, although
you should try to set them roughly right in TEM mode before
switching to STEM mode.)
If in doubt, wait until you are in scanning mode and can see
an image on the scanning monitors before you attempt to
correct astigmatism. However, note that if the stigmators
are wrong and you change them later, all the above
alignments should be re-iterated if you want the best probe
possible. Misalignment, tilt, astigmatism, defocus and
spherical aberration (which is the thing that makes the
Ronchigram look the way it does) are all just lens
aberrations that add up and affect the focus of the probe.
Change any one of these variables, and another one will no
longer be optimal.
Whatever you do, don't try turn C2 so high that what you see
on the phosphor screen reaches a focus. This is bound to
look astigmatic - but don't correct the condenser stigmators
in this condition. Set C2 at its STEM mode setting. Now
focus the objective lens: in other words, change the focus
until the Ronchigram magnification is a maximum (i.e. the
intensity distribution 'blows up' at its centre). If there
is astigmatism present, the Ronchigram will not 'blow up'
into a uniform infinite magnification blob. Instead, it
will look stretched in one direction at one setting of
objective defocus, and stretched roughly at right-angles at
another setting of defocus. Set the objective between these
two extremes, and then correct the condenser stigmators,
turning them so that whatever you see spreads more uniformly
over the Ronchigram. If the stretching of the image gets
more extreme, you're turning the stigmator the wrong way.
As a general rule, for the best scanning probe resolution,
you should select a condenser aperture size that just
selects the middle 'flat' area of intensity in the Ronchigram
when it is just in focus. 'Just in focus' means that as we
decrease focus from above the beam cross-over setting, the
very centre of the Ronchigram has just exploded into a blob,
but has not yet become a clear inverted shadow image.
Because changing spot size changes the value of C2 as well
as C1 (in STEM mode), and this affects the angle of
convergence at the specimen, the size of the condenser
aperture needed to fulfil this condition may also change.



Copyright J M Rodenburg
| 
